Surface chemistry Questions and Answers

Physical Chemistry
Surface chemistryAccording to Freundlich adsorption isotherm which of the following correct at low pressure of adsorption O E 8 x E 8 p hr m O XxP

Physical Chemistry
Surface chemistryWhich of the following is are correct regarding chemisorption OIt is irreversible OIt is highly specific O It results in unimolecular layer O All of these hr

Physical Chemistry
Surface chemistry3 Which are not purely surface phenomena ho a Adsorption surface tensione dion b Surface tension viscosity c Adsorption viscosity 4 Adsorbed acetic noi d Absorption viscosity bevis v a d 10m to 10 m

Physical Chemistry
Surface chemistryWhich of the following is correct for adsorption theory of Heterogenous catalysis Statement l This theory explains why the catalyst remains unchanged in mass and chemical composition at the end of the reaction Statement II This theory explains the action of catalytic promoters and catalytic poison Statement I is true Statement II is false Statement I is false Statement II is true Both Statement I and Statement Il are true Both Statement I and Statement II are false

Physical Chemistry
Surface chemistry3 Which gas is adsorbed to maximum amount by activated carbon a H g b He g c CO g d CO g 8 4 The volume of gases NH3 CO and H adsorbed by one gram of charcoal at 300 K are in orde of

Physical Chemistry
Surface chemistryConsider the chemisorption of N on a metal surface Identify the correct statement O The value of AH is always positive e are transferred from metal to 2p orbital of N molecule Due to adsorption of N on metal bond order between N N increases After adsorption the energy required to dissociate the N N bond will be lesser as compared to isolated N molecule

Physical Chemistry
Surface chemistry3 Which of the following has the least flocculating value for a sol whose particles move towards the cathode in the presence of an electric field 1 NaCl 3 Na PO 2 Na SO4 4 Na C O4

Physical Chemistry
Surface chemistryWhich one of the following statements is wrong about adsorption 1 It is a selective and specific process 2 It is a reversible process 3 An increase in the gaseous adsorbate causes an increase in a adsorption However at high pressure the adsorption becomes constant 1 It is an endothermic process
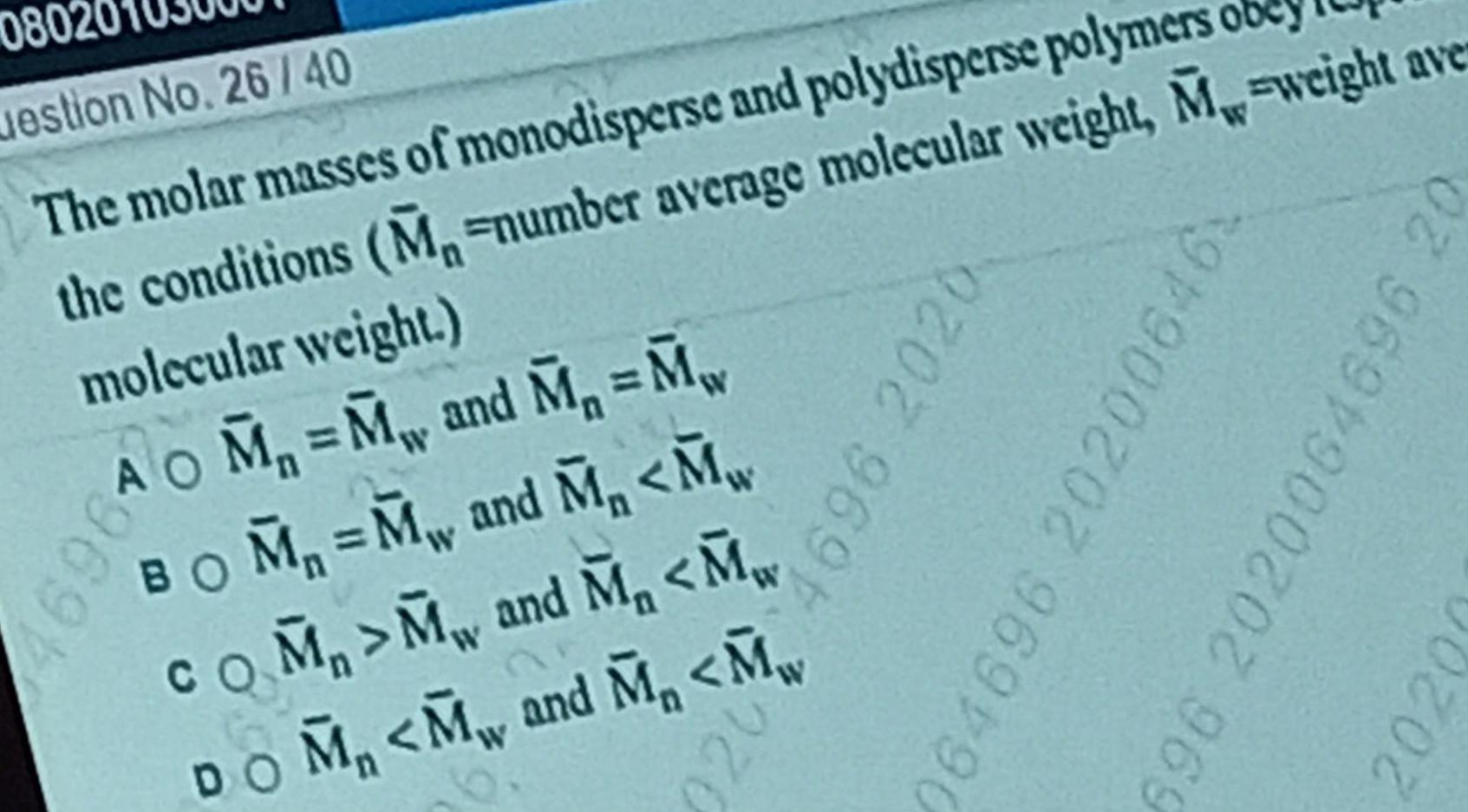
Physical Chemistry
Surface chemistry080 uestion No 26 40 The molar masses of monodisperse and polydisperse polymers obey the conditions M number average molecular weight M weight ave molecular weight 169610 M M and M M BOM M and M M COM M and M M DOM M and M M 9202 969 064696 202006 6 696 2020064696 20 2020

Physical Chemistry
Surface chemistryamount of adsorbent which of the following relations is not related to adsorption process CBSE PMT Pre 2011 X m pxT X f p at constant T m X f r at constant p m p f r at constant

Physical Chemistry
Surface chemistryTo make negative sols Agl which of the following mixin is favoured 10 ml 0 1 M AgNO3 10 ml 0 1 M KI 10 ml 0 1 M AgNO3 12 ml 0 1 M KI 12 ml 0 1 M AgNO3 8 ml 0 1 M KI hr mir All of these

Physical Chemistry
Surface chemistryets are filled with c hydrogen gas b carbon dioxide d nitrogen Identify incorrect statement from the following a During rainy season the power supply to our home from the electric pole will be interrupted due formation of metallic oxide layer on the electric wire b Vitamin E and Vitamin C are food preservatives prevent the food from spoilage c Food contianing fats and oils are stored in air tight containers or filled with Nitrogen gas d Rancidity is a reduction reaction 81

Physical Chemistry
Surface chemistryBredig s Arc method is suitable for making which of the following sol Gem stone O Gold sols Basic dye nr Gum arabic

Physical Chemistry
Surface chemistryYou have been given four charged sols Sol 1 Al O xH O Sol 2 CdS Sol Sol 3 TiO Sol Sol 4 Haemoglobin Consider the following statements S In electrophoresis the dispersed phase of only two of the given sols will move towards cathode S In electrophoresis the dispersion medium of only three of the given sols will move towards anode S3 Only three of given sols can be precipitated by K4 Fe CN 6 Which of the following options contain only the incorrect set of statement s

Physical Chemistry
Surface chemistry1 b You are now required to grow a further 0 10micron SiO on top of the 1 6micron of SiO by dry oxidation at 1130 C How long will this take NOTE Show how you used equations to determine parameters B and B A and time t 13 marks

Physical Chemistry
Surface chemistrySubstances which accelerate the rate of chemical reaction and themselves remain chemically and quantitatively unchanged after the reaction are known as catalysts The catalytic activity is localized on the surface of the catalyst The mechanism involves the following five steps Not in the order 1 Occurrence of the chemical reaction on the catalyst s surface through the formation of an intermediate II Desorption of reaction products from the catalyst surface III Adsorption of reactant molecules on the surface of the catalyst IV Diffusion of reaction products away from the catalyst s surface V Diffusion of reactant to the surface of the catalyst The correct order of sequence of the above steps to carry out a catalytic process should be V III 1 IV II V III 1 11 IV III V 1 IV II III 1 11 IV

Physical Chemistry
Surface chemistryThe dispersion medium of colloidal solution of iron III oxide and colloidal gold is negatively and positively charged respectively Identify the correct statement s Electrophoresis can cause coagulation in both the sols Mixing of both the sols will cause coagulation Less amount of sodium sulphate is required for coagulation of gold solution as compared to that required for coagulation of iron III oxide sol Calcium chloride solution coagulates the gold solution more readily than the Fe OH 3 sol

Physical Chemistry
Surface chemistryConsider the following statements regarding micelles 1 At critical micelle concentration several properties of the solution of surfactants such as molar conductivity surface tension and osmotic pressure undergo a dramatic change 2 Micelles from ionic surfactants can be formed only above a certain temperature called the Kraft temperature 3 The enthalpy of micelle formation in aqueous systems in slightly negative Which of the above state is are correct 1 alone 1 and 2 1 2 and 3 1 and 3

Physical Chemistry
Surface chemistry50 ml of 0 2 M acetic acid is shaken with 10 g activated charcoal The concentration of acetic acid is reduced to 0 5 times the original molarity The amount of solute in g adsorbed per gram of the adsorbent is approximately

Physical Chemistry
Surface chemistrySolveLancer Test Which of the following is the type of interaction present when substrate bind to enzyme SolveLancer Test 1 Ionic bonding 2 Hydrogen bonding 3 van der Waals interaction 4 dipole dipole interaction a 1 2 b 1 2 3 c 1 2 3 4 d 2 3 4

Physical Chemistry
Surface chemistry5 g of solid adsorbent is dropped in a container of 1 5 L N gas at 1 atm and 300 K The pressure of N is reduced to 20 The mass of nitrogen in g adsorbed per gram of adsorbent is Given R 0 08L atm K mol

Physical Chemistry
Surface chemistryWhich of the following is are correct about the adsorption theory of catalysis A Catalysis occur through physisorption B The increase in concentration of reactants on the surface increases the rate of reaction C The heat of adsorption is utilised in enhancing the rate of reaction D The adsorbate molecules become immobile at the catalyst surface The reaction A s 2B g is in equilibrium at 4 atm and 27 C If the volume of system

Physical Chemistry
Surface chemistry9 For real gases van der Waals equation is an V2 where a and b are van der Waals constants Two sets of gases are I 0 CO H and He CH4 O and H II The gases given in set I in increasing order of b and gases given in set II in decreasing order of a are arranged below Select the correct order from the following written as P V nb n RT a I He H CO O II CH4 H O b 1 O He H CO II H O CH4 c 1 H He O CO II CH4 O H d 1 H O He CO II O CH4 H Mains 2012 10 Equal volumes of two monatomic gases A ad ssure

Physical Chemistry
Surface chemistryWhat should be the momentum in gram centimetre per second of a particle if its de Broglie wavelength is 1 and the value of h is 6 6252 x 10 27 erg second 1 6 6252 x 10 19 gcm s 2 6 6252 x 10 21 gcm s 3 6 6252 x 10 24 gcm s 4 6 6252 x 10 27 gcm s

Physical Chemistry
Surface chemistryIn physisorption adsorbent does not show specificity for any particular gas because Involved Van der Waals forces are universal a Enthalpy of adsorption is low Gases involved behave like ideal gases It is a reversible process d O b Oc

Physical Chemistry
Surface chemistry57 The langmuir adsorption isotherm is deduced using the assumption A The adsorption sites are equivalent in their ability to adsorb the particles B The heat of adsorption varies with coverage C The adsorption molecules interact with each other D The adsorption takes place in multiplayers

Physical Chemistry
Surface chemistrystion No 28 40 collodal solution of Agl is prepared by adding AgNO solution to KI solution ull m excess then the charge on the colloidal particles will be AO Positve BO Negative CO Neutral DO None of these

Physical Chemistry
Surface chemistryReaction of Br with Na CO in aqueous solution gives sodium bromide and sodium bromate with evolution of CO gas The number of sodium bromide molecules involved in the balanced chemical equation is

Physical Chemistry
Surface chemistryIs the reaction correct and is both KOH and Mno2 colo ess Date Alknes alkynes reacts with Balyer s reagent and decolourise it s pink colour 36 Pink colourless KMn 04 H O KOH Mnozt CH CH H 0 0 CH CH2 0 OH OH H hydroxy

Physical Chemistry
Surface chemistryThe stability of lyophilic colloids is due to Colour of their particles a layer of dispersion medium on their particles the smaller size of their particles the large size of their particles

Physical Chemistry
Surface chemistryWhich of the following is correct for Langmuir s adsorption isotherm W extent of adsorption i e x m ap 1 bP A X m W Plot of P W 12 All of these a 1 versus P has as intercept a

Physical Chemistry
Surface chemistryWhich of the following phenomenon occurs when a chalk stick is dipped in ink A Adsorption of coloured substance B Adsorption of solvent C Absorption and adsorption both of solvent D Absorption of solvent

Physical Chemistry
Surface chemistryIn coagulation of a gold sol which of the following Fe CN 6 4 PO4 3 SO4 2 CI will have 1 Greatest flocculation value 2 Greatest coagulation value 3 Greatest coagulation power 4 Greatest flocculation power
Physical Chemistry
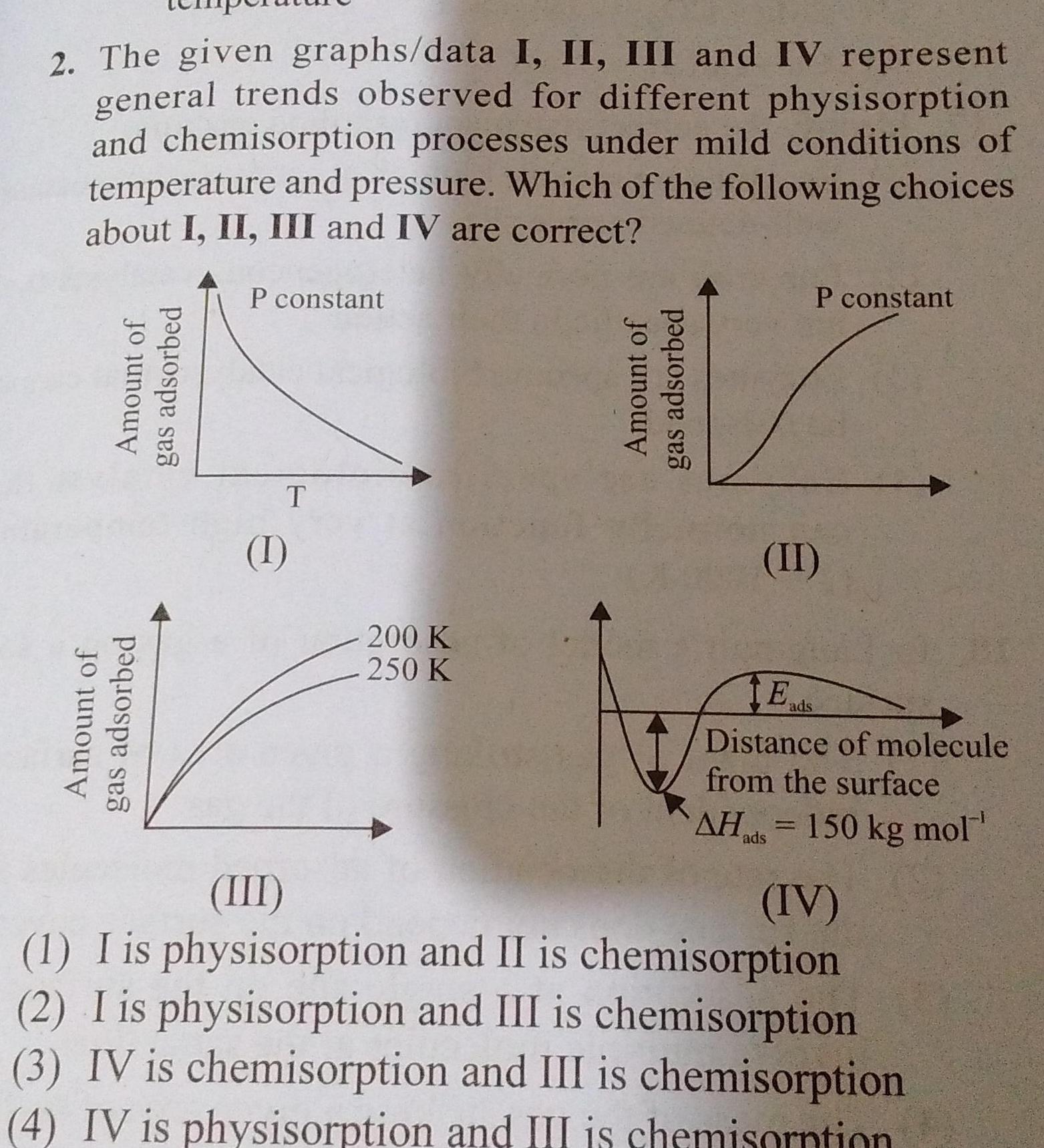
Surface chemistry2 The given graphs data I II III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and pressure Which of the following choices about I II III and IV are correct Amount of gas adsorbed Amount of gas adsorbed P constant T 1 200 K 250 K Amount of gas adsorbed P constant II E Distance of molecule from the surface AHads 150 kg mol IV ads III 1 I is physisorption and II is chemisorption 2 I is physisorption and III is chemisorption 3 IV is chemisorption and III is chemisorption 4 IV is physisorption and III is chemisorption

Physical Chemistry
Surface chemistryCount the number of correct statements i Minimum potential required for electrophoresis is called zeta potential ii Tyndall effect increases with increase in difference in size of particle and wavelength of light used iii Minimum amount of electrolyte in millimoles per 100 ml required to caus precipitate in two hours is called coagulating value iv Both physical and chemical adsorption decreases with temperature v Chemical bonds may be covalent or ionic in chemical adsorption vi Zeolites are shape selective catalyst

Physical Chemistry
Surface chemistry4 None of the above 5 I II and III are three adsorption isotherm at three temperatures T T and T where x is the amount of adsorbate adsorbed on m g of the adsorbent Temperatures T T and T3 are in the order 1 T T T3 3 T3 T T XIE m p 2 T T T3 4 No specific order T T T3

Physical Chemistry
Surface chemistry4 FeCl3 Fe 3OH 3 A sol has positively charged colloidal particles Which c the following solutions is required in lowest concentratic for coagulation 1 NaCl 3 ZnCl 2 K4 Fe CN 6 4 Na SO4

Physical Chemistry
Surface chemistrycal adsorption 1 increases with increase in temperature 2 decreases with increase in temperature 3 first increases then decreases with increase in temperature 4 first decreases then increases with increase in temperature

Physical Chemistry
Surface chemistryall Enzyme catalysis enzyme mechanism homogenous and heterogeneous catalysis and adsorption theory of heterogeneous catalysis catalyst related any chemical reaction All are example o chemical adsorption 11 17 AM

Physical Chemistry
Surface chemistryWhich of the following is correct from of Freundlich adsorption isotherm equation O XE kpl n n m kp1 n 1 kp1 n 0 1 0 0 1

Physical Chemistry
Surface chemistry12 A reddish brown sol is obtained by adding small quantity of FeCl3 solution to freshly prepared and well washed Fe OH 3 ppt Fixed layer and mobile layer are represented as 3 1 FeCl3 Fe 2 Fe OH 3 Fe 3 Fe OH 3 Fe 4 FeCl Fe 3 OH CI 3C1 3OH

Physical Chemistry
Surface chemistry3 5 Methylene blue in aqueous solution is adsorbed on activated charcoal at 25 C For this process the correct statement is 1 The adsorption is accompanied by a decrease in enthalpy 2 The adsorption requires activation at 25 C 3 The adsorption increases with increases of temperature 4 The adsorption is irreversible

Physical Chemistry
Surface chemistryQ 7 moderate O 4 1 Which of the following is true in respect of adsorption AG 0 AS 0 AH 0 O AG 0 AS 0 AH 0 O AG 0 AS 0 AH 0 00 19 O AG 0 AS 0 AH 0

Physical Chemistry
Surface chemistry14 Which statement is not true 1 pH of 1 x 108 M HCl is 8 2 96500 coulomb deposits 1 g equivalent of cop 3 Conjugate base of H PO4 is HPO 4 pH pOH 14 for all aqueous solution

Physical Chemistry
Surface chemistryQ 11 On adding 1 mL solution of 10 NaCl to 10 mL gold solution in the presence of 0 25 g of starch the coagulation is just prevented Starch has the gold number equal to O 0 25 O 2 5 4 1 00 33 250 BEL

Physical Chemistry
Surface chemistryDuring adsorption on a solid diatomic molecules of a gas X dissociate into atoms Which of the following relationships are true for the said process 0 surface coverage on a uniform adsorbent surface ka is the rate constant of adsorption and ka is the rate constant of desorption P is the pressure of gas X 8 KP 1 KP KPZ 1 1 KPZ K K K

Physical Chemistry
Surface chemistryWhich one of the following does not involve coagulation O Treatment of drinking water by potash alum O Formation of delta regions Peptization Clotting of blood by the use of ferric chloride

Physical Chemistry
Surface chemistryThe incorrect assumption regarding the Langmuir adsorption isotherm of a gas on solid surface is The adsorption sites are equivalent in their ability to adsorb the particles It is unimolecular layer in nature It involves two opposite processes i e condensation of gas molecules and evaporation of these molecules The rate of condensation of a gas is independent to the unoccupied surface adsorbent

Physical Chemistry
Surface chemistryA 1 litre solution containing equal moles of FeO and Feo 80 was titrated with 70 ml 0 3M KMnO4 in acid medium Millimoles of Fe3 produced are

Physical Chemistry
Surface chemistryThe coagulation values in millimoles per litre of the electrolyte used for the coagulation of As283 ar given below I NaCl 52 II BaCl 0 69 III MgSO4 0 22 The correct order of the coagulation power is 1 I II III 2 II I III 3 III II I 4 III I II